New Discovery: Fractal Patterns in Natural Enzymes
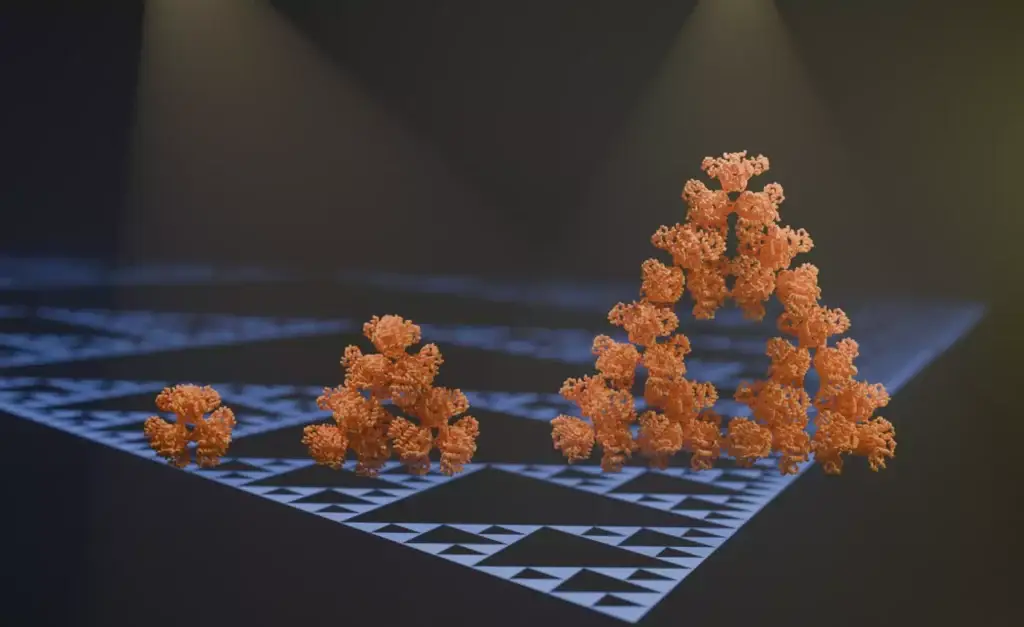
A study titled “Emergence of Fractal Geometries in the Evolution of a Metabolic Enzyme“ has been published in Nature, authored by Franziska L. Sendker and colleagues. This research unveils an extraordinary phenomenon in molecular biology: the self-assembly of a metabolic enzyme into fractal geometries, specifically Sierpiński triangles. The enzyme in question is citrate synthase from the cyanobacterium Synechococcus elongatus. This discovery marks one of the first instances where such intricate fractal patterns have been observed in naturally occurring molecular assemblies, distinguishing it from synthetic fractal geometries typically produced in laboratory settings.
Uncovering Molecular Fractals
Fractals are complex patterns characterized by self-similarity across different scales—they repeat the same basic shape regardless of magnification. While fractal patterns are common in macroscopic natural phenomena like snowflakes, coastlines, and tree branching, their presence at the microscopic molecular level is rare. The identification of fractal geometries in a naturally forming protein complex represents a significant advancement in understanding molecular self-assembly.
Using advanced imaging techniques such as cryo-electron microscopy, the researchers visualized how citrate synthase molecules assemble into higher-order structures. Initially forming hexameric units, these molecules further organize into the larger fractal patterns of Sierpiński triangles. This hierarchical assembly demonstrates the enzyme’s ability to form complex geometries through simple molecular interactions.
Evolutionary Insights
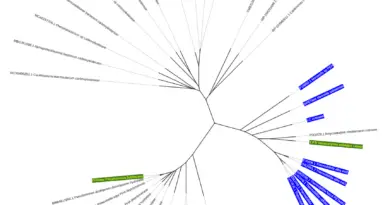
An intriguing aspect of the study is the exploration of the evolutionary pathway leading to the fractal-forming citrate synthase. By employing ancestral sequence reconstruction, the researchers traced back the enzyme’s evolutionary history. This analysis revealed that the ability to form fractal structures evolved from non-fractal predecessors through relatively minor genetic changes. Such findings suggest that complex and regulatable assemblies can emerge in proteins without direct selection for a specific function.
Despite the enzyme’s capacity to form these intricate structures, the study found no direct physiological role for the fractal assemblies within the organism. The fractal formation appears to be an evolutionary byproduct rather than a trait selected for a particular biological function. This observation highlights the unpredictable nature of evolutionary processes in shaping molecular structures.
Implications for Molecular Biology
The discovery of naturally occurring fractal geometries in a metabolic enzyme has several significant implications:
- Expanding Protein Complex Knowledge: It broadens the scope of known protein complex formations, adding a new dimension to our understanding of protein assembly.
- Insights into Self-Assembly Mechanisms: Understanding how simple molecular interactions lead to complex fractal patterns can inform the design of novel biomaterials and nanotechnology applications.
- Evolutionary Biology: The study underscores how evolutionary processes can produce complex structures without direct functional advantages, contributing to the discourse on the role of natural selection in molecular evolution.
This research by Sendker et al. not only reveals a fascinating example of molecular self-assembly but also challenges our perceptions of how complex structures can arise in biology. The emergence of fractal geometries in a metabolic enzyme exemplifies the intricate interplay between molecular form and function, even when the function may not be immediately apparent. As scientists continue to explore the depths of molecular biology, discoveries like this illuminate the extraordinary complexity inherent in natural processes.
References
- Original Research Paper. Emergence of fractal geometries in the evolution of a metabolic enzyme. Nature.
Keywords
Fractal Geometry, Citrate Synthase, Molecular Self-Assembly, Synechococcus elongatus, Cryo-Electron Microscopy, Evolutionary Biology, Protein Complexes, Sierpiński Triangle, Ancestral Sequence Reconstruction, Molecular Biology
Disclaimer: The information presented in this article is for informational purposes only and reflects the findings of the referenced study as of the publication date. For detailed information, please refer to the original research paper or consult professionals in the field.